

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:50, lindseyklewis1p56uvi
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2


Chemistry, 23.06.2019 06:30, madelineb6243
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3
You know the right answer?
In the following reaction, how many grams of oxygen will react with 10.47 grams of benzene (c6h6)?...
Questions in other subjects:


Biology, 05.04.2021 14:00

Biology, 05.04.2021 14:00



English, 05.04.2021 14:00

Mathematics, 05.04.2021 14:00

English, 05.04.2021 14:00


Mathematics, 05.04.2021 14:00


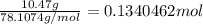
 of oxygen
of oxygen


