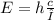Chemistry, 11.10.2019 00:00 diwashkandel6pe02af
Calculate the wavelength of a photon having energy of 1.257 x 10-24 joules. (planck’s constant is 6.626 x 10-34 joule seconds; the speed of light is 2.998 x 108 m/s)

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:31, cassiuspricerules
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 04:40, twinchristiansp4xhd2
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Calculate the wavelength of a photon having energy of 1.257 x 10-24 joules. (planck’s constant is 6....
Questions in other subjects:

Physics, 17.12.2019 16:31

Mathematics, 17.12.2019 16:31


Health, 17.12.2019 16:31

Mathematics, 17.12.2019 16:31

Mathematics, 17.12.2019 16:31


English, 17.12.2019 17:31


Mathematics, 17.12.2019 17:31