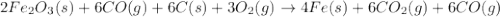Blast furnaces extract pure iron from the iron(iii) oxide in iron ore in a two step sequence. in the first step, carbon and oxygen react to form carbon monoxide: 2c (s) + o2 (g) → 2co (g)in the second step, iron(iii) oxide and carbon monoxide react to form iron and carbon dioxide: fe2o3 (s) + 3co (g) → 2fe (s) + 3co2 (g)write the net chemical equation for the production of iron from carbon, oxygen and iron(iii) oxide. be sure your equation is balanced.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, mamasmontoya
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2


Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Blast furnaces extract pure iron from the iron(iii) oxide in iron ore in a two step sequence. in the...
Questions in other subjects:



Mathematics, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01

Computers and Technology, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01

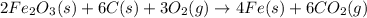
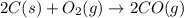 ..[1]
..[1]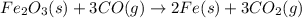 ..[2]
..[2]