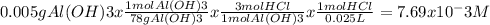Chemistry, 12.12.2019 05:31 nathanscastr02
Antacids are compounds, usually bases, used to decrease the amount of hydrochloric acid in the stomach. write a brief titration procedure for determining how many moles of acid a single antacid tablet would neutralize. this procedure should be detailed and correct so that someone could go into a laboratory and follow the procedure you have written.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1


Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
Antacids are compounds, usually bases, used to decrease the amount of hydrochloric acid in the stoma...
Questions in other subjects:

Mathematics, 23.01.2020 06:31

Mathematics, 23.01.2020 06:31



History, 23.01.2020 06:31

Chemistry, 23.01.2020 06:31