Chemistry, 12.12.2019 05:31 ninaaforever
Consider the dissolution of ammonium nitrate. 1.25 g of amminum nitrate is dissolved in enough water to make 25.0 ml of solution. the initial temperature is 25.8 degc and the final temperature (after the solid dissolves) is 21.9 degc. calculate the change in enthalpy for the reaction in kj. use 1.0 g/ml as the density of solution and 4.18 j/g*degc as the specific heat capacity.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2
You know the right answer?
Consider the dissolution of ammonium nitrate. 1.25 g of amminum nitrate is dissolved in enough water...
Questions in other subjects:


Chemistry, 03.01.2021 14:10

Mathematics, 03.01.2021 14:10


Mathematics, 03.01.2021 14:10

English, 03.01.2021 14:20


Biology, 03.01.2021 14:20

History, 03.01.2021 14:20

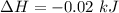
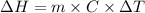
 is the enthalpy change
is the enthalpy change is the temperature change
is the temperature change