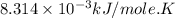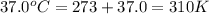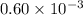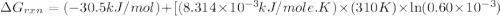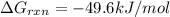Chemistry, 12.12.2019 05:31 eileentennyson
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by the reaction atp ( aq ) + h 2 o ( l ) ⟶ adp ( aq ) + hpo 2 − 4 ( aq ) for which δ g ∘ rxn = − 30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δ g rxn in a biological cell in which [ atp ] = 5.0 mm, [ adp ] = 0.60 mm, and [ hpo 2 − 4 ] = 5.0 mm.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions in other subjects:


English, 26.03.2021 02:10

Mathematics, 26.03.2021 02:10

Mathematics, 26.03.2021 02:10

Mathematics, 26.03.2021 02:10


Mathematics, 26.03.2021 02:10

Physics, 26.03.2021 02:10

History, 26.03.2021 02:10

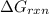 is -49.6 kJ/mol
is -49.6 kJ/mol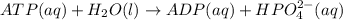
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0414/8690/ccdf0.png)
![[ATP]](/tpl/images/0414/8690/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0414/8690/68360.png) = 0.60 mM
= 0.60 mM![[HPO_4^{2-}]](/tpl/images/0414/8690/c0ca9.png) = 5.0 mM
= 5.0 mM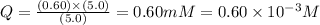
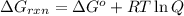 ............(1)
............(1)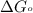 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol