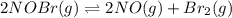Chemistry, 12.12.2019 04:31 dhurtado1195
For the following reaction, the equilibrium constant kc is 2.0 at a certain temperature. the reaction is endothermic. what do you expect to happen to the concentration of no if the temperature is doubled? 2nobr( g) ⇌ 2no( g) + br 2( g) the change in concentration of [no] will depend on the size of the vessel. the concentration of no will increase. the concentration of no will decrease. a catalyst will be needed to make a change in concentration. there will be no change in [no].

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

You know the right answer?
For the following reaction, the equilibrium constant kc is 2.0 at a certain temperature. the reactio...
Questions in other subjects:

Biology, 21.11.2020 20:30

Mathematics, 21.11.2020 20:30

English, 21.11.2020 20:30

Social Studies, 21.11.2020 20:30

English, 21.11.2020 20:30

SAT, 21.11.2020 20:30

History, 21.11.2020 20:30

Chemistry, 21.11.2020 20:30

Geography, 21.11.2020 20:30

Mathematics, 21.11.2020 20:30