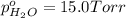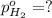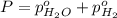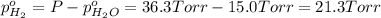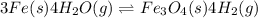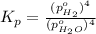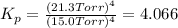Chemistry, 12.12.2019 04:31 makalaily9342
In a study of the following reaction at 1200 k it was observed that when the equilibrium partial pressure of water vapor is 15.0 torr, the total pressure at equilibrium is 36.3 torr. 3 fe(s) 4 h2o(g) equilibrium reaction arrow fe3o4(s) 4 h2(g) calculate the value of kp for this reaction at 1200 k. hint: apply dalton's law of partial pressures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, tot92
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
In a study of the following reaction at 1200 k it was observed that when the equilibrium partial pre...
Questions in other subjects:


Mathematics, 06.10.2019 09:01


Social Studies, 06.10.2019 09:01

Mathematics, 06.10.2019 09:01

Geography, 06.10.2019 09:01

Mathematics, 06.10.2019 09:01

Chemistry, 06.10.2019 09:01


Mathematics, 06.10.2019 09:01

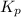 for this reaction at 1200 K is 4.066.
for this reaction at 1200 K is 4.066.