Chemistry, 12.12.2019 03:31 ayoismeisjjjjuan
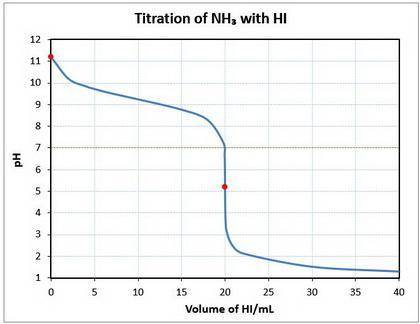
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0 ml of a solution of 0.150 mol/l of the strong acid hydroiodic acid (hi (
a) write a balanced equation for the titration reaction.
b) what is the ph of the ammonia solution before the titration begins?
c) what is the ph at the equivalence point?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, haybaby312oxdjli
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1


Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0...
Questions in other subjects:

Physics, 22.01.2021 18:50

History, 22.01.2021 18:50


Geography, 22.01.2021 18:50


Mathematics, 22.01.2021 18:50




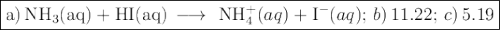


![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.150 - x} = 1.8 \times 10^{-5}](/tpl/images/0414/6924/2256c.png)

![\dfrac{x^{2}}{0.150} = 1.8 \times 10^{-5}\\\\x^{2} = 0.150 \times 1.8 \times 10^{-5}\\x^{2} = 2.7 \times 10^{-6}\\x = \sqrt{2.7 \times 10^{-6}}\\x = \text{[OH]}^{-} = 1.64 \times 10^{-3} \text{ mol/L}](/tpl/images/0414/6924/0b94c.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(1.64 \times 10^{-3}) = 2.78\\\\\text{pH} = 14.00 - \text{pOH} = 14.00 - 2.78 = \mathbf{11.22}\\\\\text{The pH of the solution at equilibrium is } \large \boxed{\mathbf{11.22}}](/tpl/images/0414/6924/f4986.png)

![\rm [BH^{+}] = \dfrac{\text{3.00 mmol}}{\text{40.00 mL}} = \text{0.0750 mol/L}](/tpl/images/0414/6924/607b2.png)

![\dfrac{x^{2}}{0.0750} = 5.56 \times 10^{-10}\\\\x^{2} = 0.0750 \times 5.56 \times 10^{-10}\\x^{2} = 4.17 \times 10^{-11}\\x = \sqrt{4.17 \times 10^{-11}}\\\rm [H_{3}O^{+}] =x = 6.46 \times 10^{-6}\, mol \cdot L^{-1}](/tpl/images/0414/6924/dc4d9.png)
![\text{pH} = -\log{\rm[H_{3}O^{+}]} = -\log{6.46 \times 10^{-6}} = \large \boxed{\mathbf{5.19}}](/tpl/images/0414/6924/b2d28.png)