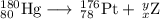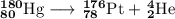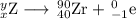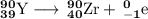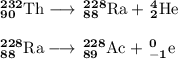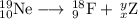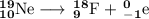Chemistry, 12.12.2019 02:31 brooke012002
Write a balanced equation for each of the following nuclear reactions: (a) mercury-180 decays into platinum-176 (b) zirconium-90 and an electron are produced by the decay of an unstable nucleus (c) thorium-232 decays and produces an alpha particle and a radium-228 nucleus, which decays into actinium-228 by beta decay (d) neon-19 decays into fluorine-19

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

You know the right answer?
Write a balanced equation for each of the following nuclear reactions: (a) mercury-180 decays into...
Questions in other subjects:



Biology, 04.03.2021 09:20



Mathematics, 04.03.2021 09:20

Mathematics, 04.03.2021 09:20

Computers and Technology, 04.03.2021 09:20


English, 04.03.2021 09:20

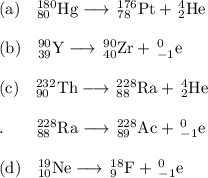
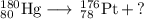
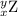 , where x = the atomic number, y = the mass number, and Z = the symbol of the element .
, where x = the atomic number, y = the mass number, and Z = the symbol of the element .