
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0 torr, 13.2 torr, and 13.2 torr, respectively. a quantity of cl 2 ( g ) is injected into the mixture, and the total pressure jumps to 263.0 torr. the appropriate chemical equation is pcl 3 ( g ) + cl 2 ( g ) − ⇀ ↽ − pcl 5 ( g ) calculate the new partial pressures after equilibrium is reestablished.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 23.06.2019 12:50, ttangelique
What is the relative mass of an electron? a) 1/1840 the mass of a neutron + proton b) 1/1840 the mass of an alpha particle c) 1/1840 the mass of a c-12 atom d) 1/1840 the mass of a hydrogen atom
Answers: 3
You know the right answer?
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0...
Questions in other subjects:


English, 21.08.2019 16:30



English, 21.08.2019 16:30

Mathematics, 21.08.2019 16:30


Chemistry, 21.08.2019 16:30


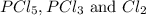 when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.
when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.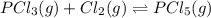
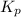 for above reaction follows:
for above reaction follows: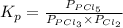 ........(1)
........(1)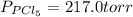
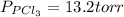
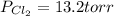
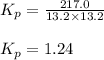
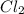 .
.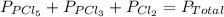
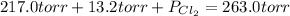
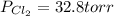
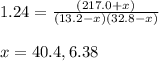
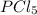 = (217.0+x) = (217.0+6.38) = 223.4 torr
= (217.0+x) = (217.0+6.38) = 223.4 torr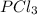 = (13.2-x) = (13.2-6.38) = 6.82 torr
= (13.2-x) = (13.2-6.38) = 6.82 torr

