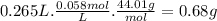Chemistry, 12.12.2019 00:31 ShlomoShekelstein
The formula that governs the concentration of gas dissolved in a solvent based on its pressure is given by henry's law c kp where: c solubility of a gas at a fixed temperature (in mol/l, m) k henry's law constant (in units of mol/l atm) p partial pressure of the gas (in units of atm).how many grams of carbon dioxide gas is dissolved in a 265 ml can of cola if the manufacturer uses a pressure of 1.7 atm in the bottling process at 25 °c? assume that the k of co2 in water = 0.034 mol/l·atm) at 25 °c. report your answer to two significant figures.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1
You know the right answer?
The formula that governs the concentration of gas dissolved in a solvent based on its pressure is gi...
Questions in other subjects:



Mathematics, 02.12.2021 16:40

History, 02.12.2021 16:40

Business, 02.12.2021 16:40


English, 02.12.2021 16:40


Mathematics, 02.12.2021 16:40

Mathematics, 02.12.2021 16:40