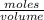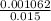Chemistry, 11.12.2019 23:31 asndjsd9397
Asolution of sodium hydroxide, 0.0500 m, is used to titrate a 15.00 ml sample of hydrochloric acid to the endpoint. the initial buret reading was 3.87 m; the final reading was 25.11 ml. what is the molarity of the acid solution.?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Asolution of sodium hydroxide, 0.0500 m, is used to titrate a 15.00 ml sample of hydrochloric acid t...
Questions in other subjects:

Arts, 04.08.2019 08:00

History, 04.08.2019 08:00

English, 04.08.2019 08:00



History, 04.08.2019 08:00