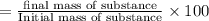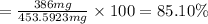Chemistry, 11.12.2019 23:31 Mypasswordishotdog11
Suppose an aspirin™ tablet contains 7 grains of the active ingredient, acetylsalicylic acid (1 grain = 64.7989 mg). if your tablet had a mass of 516 mg, calculate the percent recovery if you isolated 386 mg of acetylsalicylic acid in this experiment.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 23.06.2019 00:30, quintink
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
Suppose an aspirin™ tablet contains 7 grains of the active ingredient, acetylsalicylic acid (1 grain...
Questions in other subjects:


Mathematics, 05.03.2020 08:17