Chemistry, 11.12.2019 23:31 Dennismommie
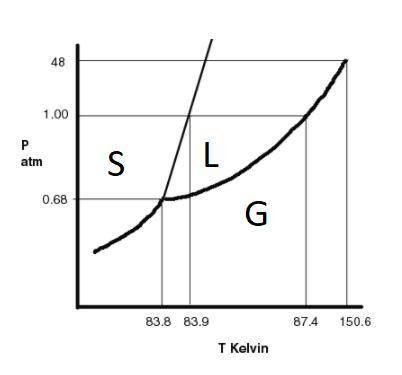
The substance argon has the following properties: normal melting point: 83.9 k normal boiling point: 87.4 k triple point: 0.68 atm, 83.8 k critical point: 48 atm, 150.6 k a sample of argon is initially at a pressure of 49.6 atm and a temperature of 101.4 k. the pressure on the sample is reduced to 0.680 atm at a constant temperature of 101.4 k. which of the following are true? choose all that apply the final state of the substance is a gas. the gas initially present will solidify. the final state of the substance is a solid. the sample is initially a liquid. one or more phase changes will occur.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, girlchamp654
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2


Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
The substance argon has the following properties: normal melting point: 83.9 k normal boiling poin...
Questions in other subjects:


History, 01.08.2019 07:30

Chemistry, 01.08.2019 07:30


Mathematics, 01.08.2019 07:30