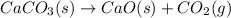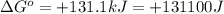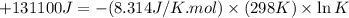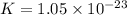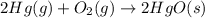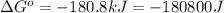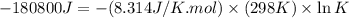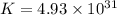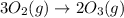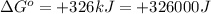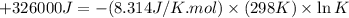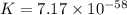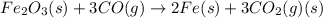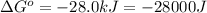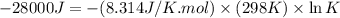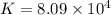Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 21:30, roserose3098
Read the chemical equation. n2 + 3h2 → 2nh3 using the volume ratio, determine how many liters of nh3 is produced if 3.6 liters of h2 reacts with an excess of n2, if all measurements are taken at the same temperature and pressure? 5.4 liters 2.4 liters 1.8 liters 1.2 liters
Answers: 1
You know the right answer?
Which of the following reactions will have the largest value of k at 298 k? a) caco3(s) → cao(s) +...
Questions in other subjects:

Mathematics, 20.05.2020 05:02



Biology, 20.05.2020 05:02

Social Studies, 20.05.2020 05:02


Mathematics, 20.05.2020 05:02



Mathematics, 20.05.2020 05:02

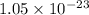
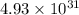
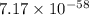
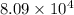
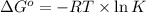
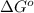 = standard Gibbs free energy
= standard Gibbs free energy