
Chemistry, 11.12.2019 20:31 brainist71
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of iodine. i2(g) equilibrium reaction arrow 2 i(g); kc = 1.35 ✕ 10−3 suppose this reaction is initiated in a 3.7 l container with 0.063 mol i2 at 970 k. calculate the concentrations of i2 and i at equilibrium.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, cynthiagutierrez65
Where can i find naap lab answers sheet/key?
Answers: 1

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of i...
Questions in other subjects:

Physics, 17.12.2019 15:31




Biology, 17.12.2019 15:31

Physics, 17.12.2019 15:31

Mathematics, 17.12.2019 15:31



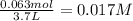
![Kc=1.35 \times 10^{-3} =\frac{[I]^{2} }{[I_{2}]} =\frac{(2x)^{2} }{(0.017-x)} \\4x^{2} +1.35 \times 10^{-3}x - 2.3 \times 10^{-5}](/tpl/images/0413/9700/35688.png)


