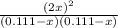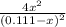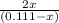Chemistry, 11.12.2019 19:31 ImBADatmath8743
The equilibrium constant for the reaction of fluorine gas with bromine gas at 300 k is 54.7 and the reaction is: br2(g) + f2(g) ⇔ 2 brf(g) what is the equilibrium concentration of fluorine if the initial concentrations of bromine and fluorine were 0.111 moles/liter in a sealed container and no product was present initially?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 19:50, nikoidurrant
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
The equilibrium constant for the reaction of fluorine gas with bromine gas at 300 k is 54.7 and the...
Questions in other subjects:



Social Studies, 19.07.2020 14:01

English, 19.07.2020 14:01



Biology, 19.07.2020 14:01

Physics, 19.07.2020 14:01

English, 19.07.2020 14:01

Business, 19.07.2020 14:01

![\frac{[BrF ]^{2} }{[ F_{2} ][Br_{2} ]}](/tpl/images/0413/8603/382cd.png)