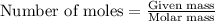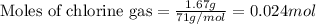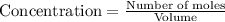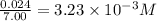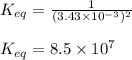Titanium and chlorine react to form titanium(iv) chloride, like this: ti(s) + 2 cl 2(g)-ticl 4( at a certain temperature, a chemist finds that a 7.0 l reaction vessel containing a mixture of titanium, chlorine, and titanium(iv) chloride at equilibrium has the following composition compound amount 1.67 g cl 2.93 g tici 2.02 g ti calculate the value of the equilibrium constant k for this reaction. round your answer to 2 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, genyjoannerubiera
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1


Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Titanium and chlorine react to form titanium(iv) chloride, like this: ti(s) + 2 cl 2(g)-ticl 4( at...
Questions in other subjects:

Law, 16.09.2019 23:30

Chemistry, 16.09.2019 23:30

Mathematics, 16.09.2019 23:30


Mathematics, 16.09.2019 23:30

Biology, 16.09.2019 23:30



Chemistry, 16.09.2019 23:40

Mathematics, 16.09.2019 23:40


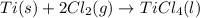
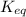 for the above reaction follows:
for the above reaction follows:![K_{eq}=\frac{1}{[Cl_2]^2}](/tpl/images/0413/8706/8ac7b.png)