
The 235u isotope (atomic mass = 235.00) undergoes fission when bombarded with neutrons. however, its natural abundance is only 0.72 percent. to separate it from the more abundant 238u isotope (atomic mass = 238.00), uranium is first converted to uf6, which is easily vaporized above room temperature. the mixture of 235uf6 and 238uf6 gases is then subjected to many stages of effusion. calculate how much more quickly 235uf6 effuses than 238uf6. give the answer as the ratio of rates of 235uf6 to 238uf6 to four decimal places.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
The 235u isotope (atomic mass = 235.00) undergoes fission when bombarded with neutrons. however, its...
Questions in other subjects:






Mathematics, 24.10.2020 06:40



Mathematics, 24.10.2020 06:40

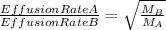
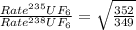 = 1.0043
= 1.0043


