
Chemistry, 11.12.2019 05:31 daemonacoster
Arrange the following solutions in order of decreasing freezing point: 0.20 m baf2, 0.15 m c6h12, 0.10 m crbr3, 0.15 m ch3ch2ch2cooh, 0.35 m kbr. (enter just the formulas in your answer. use the appropriate < , =, or > symbol to separate substances in the list.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, iloveballet1857
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1


Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Arrange the following solutions in order of decreasing freezing point: 0.20 m baf2, 0.15 m c6h12, 0...
Questions in other subjects:

Physics, 05.11.2019 13:31

World Languages, 05.11.2019 13:31

History, 05.11.2019 13:31



Mathematics, 05.11.2019 13:31




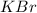 > 0.20 m
> 0.20 m 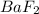 > 0.10 m
> 0.10 m 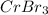 > 0.15 m
> 0.15 m 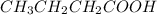 > 0.15 m
> 0.15 m 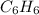
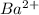 and
and 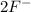
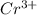 and
and 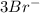
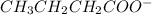 and
and 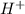 .
.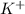 and
and 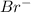 .
.

