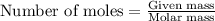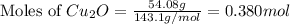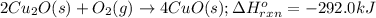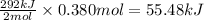Chemistry, 11.12.2019 05:31 Lydiac8715
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic process. 2 cu 2 o ( s ) + o 2 ( g ) ⟶ 4 cuo ( s ) δ h ∘ rxn = − 292.0 kj mol calculate the energy released as heat when 54.08 g cu 2 o ( s ) undergo oxidation at constant pressure.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1


Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic p...
Questions in other subjects:

Mathematics, 13.10.2019 22:00


Social Studies, 13.10.2019 22:00

Advanced Placement (AP), 13.10.2019 22:00


Mathematics, 13.10.2019 22:00


Mathematics, 13.10.2019 22:00


Mathematics, 13.10.2019 22:00

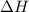 for the reaction will be -55.48 kJ
for the reaction will be -55.48 kJ