
A41.1 g sample of solid co2 (dry ice) is added to a container at a temperature of 100 k with a volume of 3.4 l. a. if the container is evacuated (all of the gas removed), sealed, and then allowed to warm to room temperature t = 298 k so that all of the solid co2 is converted to a gas, what is the pressure inside the container?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
You know the right answer?
A41.1 g sample of solid co2 (dry ice) is added to a container at a temperature of 100 k with a volum...
Questions in other subjects:


Mathematics, 11.10.2020 21:01

Mathematics, 11.10.2020 21:01

History, 11.10.2020 21:01



English, 11.10.2020 21:01


Mathematics, 11.10.2020 21:01

English, 11.10.2020 21:01

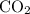 :
: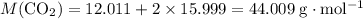 .
.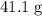 sample of
sample of  .
.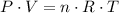 ,
, 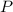 is the pressure inside the container.
is the pressure inside the container.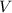 is the volume of the container.
is the volume of the container.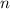 is the number of moles of particles (molecules, or atoms in case of noble gases) in the gas.
is the number of moles of particles (molecules, or atoms in case of noble gases) in the gas. is the ideal gas constant.
is the ideal gas constant. 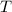 is the absolute temperature of the gas.
is the absolute temperature of the gas.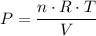 .
.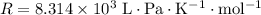 .
.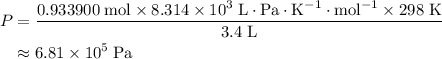 .
.

