
Chemistry, 11.12.2019 04:31 maguilarz2005
How much heat (in kj) is needed to convert 856 g of ice at −10.0°c to steam at 126.0°c? (the specific heats of ice, water, and steam are 2.03 j/g · °c, 4.184 j/g · °c, and 1.99 j/g · °c, respectively. the heat of fusion of water is 6.01 kj/mol, the heat of vaporization is 40.79 kj/mol.)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
How much heat (in kj) is needed to convert 856 g of ice at −10.0°c to steam at 126.0°c? (the specif...
Questions in other subjects:


Mathematics, 26.02.2021 22:20



Mathematics, 26.02.2021 22:20



Biology, 26.02.2021 22:20


Mathematics, 26.02.2021 22:20

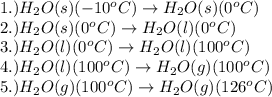
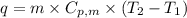 .......(1)
.......(1)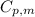 = specific heat capacity of medium
= specific heat capacity of medium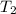 = final temperature
= final temperature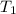 = initial temperature
= initial temperature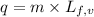 ......(2)
......(2)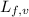 = latent heat of fusion or vaporization
= latent heat of fusion or vaporization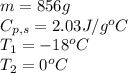
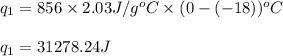
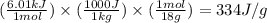
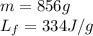
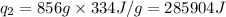
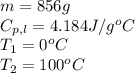
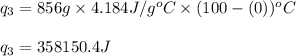
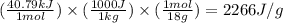
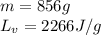
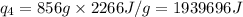
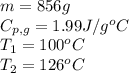
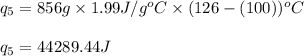
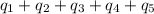
![[31278.24+285904+358150.4+1939696+44289.44]J=2659318.08J=2659.3kJ](/tpl/images/0412/9728/2c085.png)


