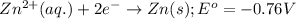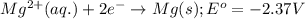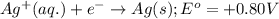Consider the following standard reduction potentials: zn2+(aq) + 2 e− latex: \longrightarrow⟶ zn(s) eo = −0.76 v mg2+(aq) + 2 e− latex: \longrightarrow⟶ mg(s) eo = −2.37 v ag+(aq) + e− latex: \longrightarrow⟶ ag(s) eo = +0.80 v which is the strongest oxidizing agent? group of answer choices

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 06:30, shateece
(04.05 hc) analyze the given diagram of the carbon cycle below. an image of carbon cycle is shown. the sun, a cloud, two trees, one on the left and the other on the right, an animal, lake, and a factory are shown in the image. an arrow is shown from the sun towards the left tree marked a. the sun is marked b. there is an arrow from the air above the clouds, marked c, towards the left tree. an arrow from a location close to the ground marked d points towards dead organisms, which is a label under the animal. an arrow marked e points from the right tree straight up to the clouds. an arrow marked f points from the animal straight up to the clouds. an arrow marked g points from the factory towards the air above the clouds, c. there is an arrow pointing from the air to the lake labeled carbonates in water, an arrow pointing down from dead organisms to fossils and fossil fuels, and an arrow from fossils to the factory. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer.
Answers: 2

Chemistry, 23.06.2019 14:20, baler19
Timed ! in which of these statements are protons, electrons, and neutrons correctly compared? quarks are present in protons and neutrons but not in electrons. quarks are present in protons, neutrons, and electrons. quarks are present in neutrons and electrons but not in protons. quarks are present in protons and electrons but not in neutrons.
Answers: 1

Chemistry, 23.06.2019 15:30, Marliii363782
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
Consider the following standard reduction potentials: zn2+(aq) + 2 e− latex: \longrightarrow⟶ zn(s...
Questions in other subjects:

Mathematics, 30.03.2021 18:00

Mathematics, 30.03.2021 18:00

English, 30.03.2021 18:00

Social Studies, 30.03.2021 18:00

History, 30.03.2021 18:00

English, 30.03.2021 18:00



Mathematics, 30.03.2021 18:00

Mathematics, 30.03.2021 18:00

 potential will always get reduced and will undergo reduction reaction easily.
potential will always get reduced and will undergo reduction reaction easily.