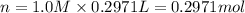Chemistry, 11.12.2019 02:31 savannahvargas512
Which of the following solutions contains the smallest number of ions? a. 156.5 ml of 2.0 m iron(iii) chloride b. 521.4 ml of 1.0 m lithium nitrate c. 374.3 ml of 2.0 m potassium hydroxide d. at least two of the above contain the smallest number of ions. e. 297.1 ml of 1.0 m sodium sulfate

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1


Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 06:00, fjsdfj1284
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
Which of the following solutions contains the smallest number of ions? a. 156.5 ml of 2.0 m iron(ii...
Questions in other subjects:

Mathematics, 13.05.2021 20:20



Mathematics, 13.05.2021 20:20


Chemistry, 13.05.2021 20:20

Mathematics, 13.05.2021 20:20


English, 13.05.2021 20:20