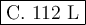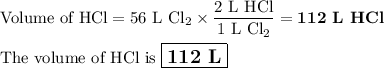Consider this reaction:
h2 (g) + cl2 (g) → 2 hcl (g)
how many liters of hcl are produce...

Chemistry, 11.12.2019 02:31 yashirachevalier
Consider this reaction:
h2 (g) + cl2 (g) → 2 hcl (g)
how many liters of hcl are produced when 56 l of chlorine are reacted with excess
hydrogen?
(one mole of any gas occupies 22.4 l under certain conditions of temperature and
pressure. assume those conditions for this question.)
a. 22.4l
b. 56 l
c. 112
d. 224 l

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1


Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 18.12.2020 01:10

History, 18.12.2020 01:10

Chemistry, 18.12.2020 01:10

English, 18.12.2020 01:10




Mathematics, 18.12.2020 01:10


Mathematics, 18.12.2020 01:10