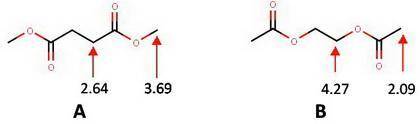
Select the single best answer. compound a exhibits two singlets in its 1h nmr spectrum at 2.64 and 3.69 ppm and the ratio of the absorbing signals is 2: 3. compound b exhibits two singlets in its 1h nmr spectrum at 2.09 and 4.27 ppm and the ratio of the absorbing signals is 3: 2. which compound corresponds to dimethyl succinate and which compound corresponds to ethylene diacetate? 1455a 1455b dimethyl succinate ethylene diacetate compound a is dimethyl succinate; compound b is ethylene diacetate. compound a is ethylene diacetate; compound b is dimethyl succinate.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Select the single best answer. compound a exhibits two singlets in its 1h nmr spectrum at 2.64 and 3...
Questions in other subjects:

English, 20.10.2020 04:01

History, 20.10.2020 04:01



Computers and Technology, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

SAT, 20.10.2020 04:01

History, 20.10.2020 04:01