Calculate the enthalpy of reaction for 2co + o2 → 2co2. given the following bond energies:
b...

Chemistry, 10.12.2019 19:31 cdjeter12oxoait
Calculate the enthalpy of reaction for 2co + o2 → 2co2. given the following bond energies:
be(c≡o) = 1074 kj/mol
be(o≡o) = 499 kj/mol
be(c≡o) = 802 kj/mol

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 22:30, needhelpasap8957
Why is the bottom layer of a trophic pyrimid the
Answers: 2
You know the right answer?
Questions in other subjects:


English, 18.03.2021 03:10





Mathematics, 18.03.2021 03:10

Spanish, 18.03.2021 03:10


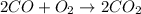
![\Delta H=[(2\times B.E_{C\equiv O})+(1\times B.E_{O\equiv O})]-[2\times B.E_{C=O}]](/tpl/images/0412/0179/8905d.png)
 = 1074 kJ/mol
= 1074 kJ/mol = 499 kJ/mol
= 499 kJ/mol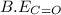 = 802 kJ/mol
= 802 kJ/mol![\Delta H=[(2\times 1074kJ/mol)+(1\times 499kJ/mol)]-[2\times 802kJ/mol]](/tpl/images/0412/0179/c40d4.png)



