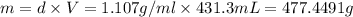A12.0 wt% solution of cacl2 (110.98 g/mol) has a density of 1.107 g/ml.
a) what is the mass (in milligrams) of a 15.0-ml solution of 12.0 wt% cacl2?
b) what is the mass (in grams) of cacl2 in 431.3 ml of a 12.0 wt% solution of cacl2?
c) what is the formal concentration of cacl2 (in molarity) of the 431.3-ml cacl2 solution described in part b?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 04:40, twinchristiansp4xhd2
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 05:00, pmbeachy3102
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
A12.0 wt% solution of cacl2 (110.98 g/mol) has a density of 1.107 g/ml.
a) what is the m...
a) what is the m...
Questions in other subjects:


Biology, 25.07.2019 11:10


Business, 25.07.2019 11:10

History, 25.07.2019 11:10

History, 25.07.2019 11:10

Mathematics, 25.07.2019 11:10