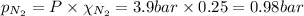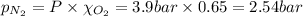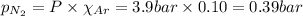Answers: 2


Other questions on the subject: Chemistry



Chemistry, 21.06.2019 20:00, Britny2386
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1
You know the right answer?
Amixture of n2, o2, and ar has mole fractions of 0.25, 0.65, and 0.10, respectively. what is the pre...
Questions in other subjects:


Mathematics, 08.02.2021 17:50

English, 08.02.2021 17:50


English, 08.02.2021 17:50



Biology, 08.02.2021 17:50

Biology, 08.02.2021 18:00

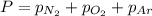
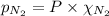
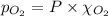
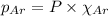
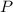 = total pressure = 3.9 bar
= total pressure = 3.9 bar 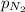 = partial pressure of nitrogen gas
= partial pressure of nitrogen gas 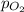 = partial pressure of oxygen gas
= partial pressure of oxygen gas 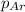 = partial pressure of argon gases
= partial pressure of argon gases 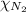 = Mole fraction of nitrogen gas = 0.25
= Mole fraction of nitrogen gas = 0.25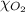 = Mole fraction of oxygen gas = 0.65
= Mole fraction of oxygen gas = 0.65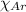 = Mole fraction of argon gases = 0.10
= Mole fraction of argon gases = 0.10