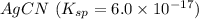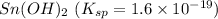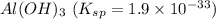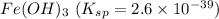Chemistry, 10.12.2019 04:31 coltonduggan
What is the most soluble salt of the following set? what is the most soluble salt of the following set?
(a) sn(oh)2 with ksp = 1.6 × 10-19
(b) al(oh)3 with ksp = 1.9 × 10-33
(c) agcn with ksp = 6.0 × 10-17
(d) fe(oh)3 with ksp = 2.6 × 10-39

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 05:40, Queenquestion9130
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
What is the most soluble salt of the following set? what is the most soluble salt of the following...
Questions in other subjects:

Physics, 09.12.2020 22:40


Social Studies, 09.12.2020 22:40

Mathematics, 09.12.2020 22:40


Mathematics, 09.12.2020 22:40