
Chemistry, 10.12.2019 02:31 Maelynne8515
Consider the reaction, cl2 + h2s => 2 hcl + s, which is found to be first order in cl2. which step of the proposed mechanism must be slow in order to agree with this rate law? cl2 => 2 cl cl + h2s => hcl + hs cl + hs => hcl + s
a. only 3
b. only 2
c. 1
d. either 2 or 3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Consider the reaction, cl2 + h2s => 2 hcl + s, which is found to be first order in cl2. which st...
Questions in other subjects:



English, 27.06.2019 21:10


Mathematics, 27.06.2019 21:10




Mathematics, 27.06.2019 21:10

Mathematics, 27.06.2019 21:10

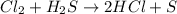
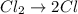
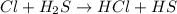
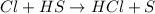
![rate=k[Cl_{2}]^\frac{1}{2}[H_{2}S]](/tpl/images/0410/9890/7d770.png)


