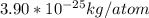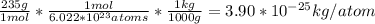Find the mass of one atom of uranium-235. recall that the mass in atomic mass units is equal to the mass in grams of one mole of atoms. avagadro's number, 6.022×1023atoms/mole, gives the number of atoms in one mole. give your answer in kilograms to three significant figures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, colochaortiz20p7cajw
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

You know the right answer?
Find the mass of one atom of uranium-235. recall that the mass in atomic mass units is equal to the...
Questions in other subjects:


Mathematics, 12.02.2021 07:30

Mathematics, 12.02.2021 07:30

Mathematics, 12.02.2021 07:30

Mathematics, 12.02.2021 07:30


Mathematics, 12.02.2021 07:30


Mathematics, 12.02.2021 07:30