Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:50, kelli151
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2


Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

You know the right answer?
When 5.00 g of solid barium react with chlorine gas to produce solid barium chloride, 31.1 kj of hea...
Questions in other subjects:


Business, 02.02.2021 08:20



Biology, 02.02.2021 08:20

Mathematics, 02.02.2021 08:20

English, 02.02.2021 08:20



Mathematics, 02.02.2021 08:20

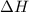 for the reaction will be -854.4 kJ
for the reaction will be -854.4 kJ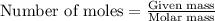
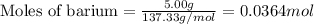
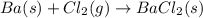
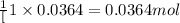 of barium chloride
of barium chloride