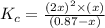Consider the following generic reaction. a 2 b ( g ) − ⇀ ↽ − 2 a ( g ) + b ( g ) the initial concentration of a 2 b is 0.87 m and the initial concentrations of a and b are assumed to be zero. what is the equilibrium constant expression ( k c ) , if the equilibrium concentration of b is represented by x ?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Consider the following generic reaction. a 2 b ( g ) − ⇀ ↽ − 2 a ( g ) + b ( g ) the initial concent...
Questions in other subjects:


Chemistry, 03.08.2019 19:30

English, 03.08.2019 19:30


History, 03.08.2019 19:30


Health, 03.08.2019 19:30

Computers and Technology, 03.08.2019 19:30


English, 03.08.2019 19:30

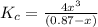
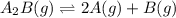
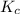 will be,
will be,![K_c=\frac{[A]^2[B]}{[A_2B]}](/tpl/images/0410/8880/c0f0a.png)