
Chemistry, 10.12.2019 01:31 bonetanne7171
Consider a solution containing 3.54 mm of an analyte, x, and 1.23 mm of a standard, s. upon chromatographic separation of the solution peak areas for x and s are 3275 and 10829, respectively. determine the response factor for x relative to s. f=to determine the concentration of x in an unknown solution, 1.00 ml of 8.27 mm s was added to 5.00 ml of the unknown x solution and the mixture was diluted to 10.0 ml. after chromatographic separation, this solution gave peak areas of 5389 and 4627 for x and s, respectively. determine the concentration of s in the 10.0 ml solution.[s]=determine the concentration of x in the 10.0 ml solution.[x]=determine the concentration of x in the unknown solution.[x]=

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, smm1106
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral. a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1


Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Consider a solution containing 3.54 mm of an analyte, x, and 1.23 mm of a standard, s. upon chromato...
Questions in other subjects:

Mathematics, 12.09.2021 02:10

English, 12.09.2021 02:10

Mathematics, 12.09.2021 02:10

Computers and Technology, 12.09.2021 02:10

History, 12.09.2021 02:10

Mathematics, 12.09.2021 02:10

Mathematics, 12.09.2021 02:10

Mathematics, 12.09.2021 02:10

Mathematics, 12.09.2021 02:10

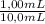 = 0,827mM
= 0,827mM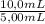 = 18,3mM
= 18,3mM


