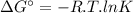Chemistry, 10.12.2019 01:31 mariahdelossantos031
The free energy change δgt at 2400 k is equal to 1.22 x 10^5j/mol. calculate the equilibrium constant at 2400 k. express your answer numerically.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
The free energy change δgt at 2400 k is equal to 1.22 x 10^5j/mol. calculate the equilibrium constan...
Questions in other subjects:







Mathematics, 14.02.2022 19:50

Mathematics, 14.02.2022 19:50

Mathematics, 14.02.2022 19:50

Mathematics, 14.02.2022 19:50