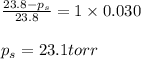Chemistry, 10.12.2019 01:31 romaguera06
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250.0 ml of water. the vapor pressure of pure water at 25°c is 23.8 torr.70.8 torr7.29 torr72.9 torr22.9 torr23.1 torr

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250....
Questions in other subjects:



Mathematics, 05.05.2020 18:35




Mathematics, 05.05.2020 18:35



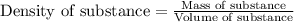
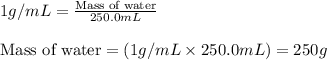
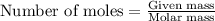 .....(1)
.....(1)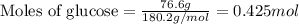
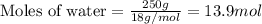
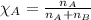
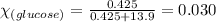
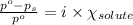
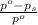 = relative lowering in vapor pressure
= relative lowering in vapor pressure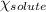 = mole fraction of solute = 0.030
= mole fraction of solute = 0.030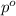 = vapor pressure of pure water = 23.8 torr
= vapor pressure of pure water = 23.8 torr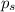 = vapor pressure of solution = ?
= vapor pressure of solution = ?