

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1


You know the right answer?
Given that kp = 3.5 x 10-4 for the reaction 2 co(g) < => c(graphite) + co2(g), what is the pa...
Questions in other subjects:




Mathematics, 17.11.2020 17:10






Mathematics, 17.11.2020 17:10

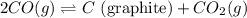
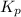 for above reaction follows:
for above reaction follows:

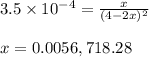
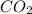 = 0.0056 atm
= 0.0056 atm

