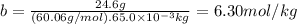Acertain substance condenses at a temperature of 123.3°c . but if a 65.0 gm sample of x is prepared with 24.6g of urea ((nh2)2co) dissolved in it, the sample is found to have a condensation point of 124.3°c instead.
1. calculate the molal boiling point elevation constant kb of x . round your answer to significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3


Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Acertain substance condenses at a temperature of 123.3°c . but if a 65.0 gm sample of x is prepared...
Questions in other subjects:



Chemistry, 04.08.2020 14:01



History, 04.08.2020 14:01


Mathematics, 04.08.2020 14:01