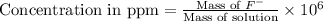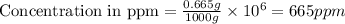Chemistry, 09.12.2019 23:31 camiloriveraveoxbgd6
Express the concentration of a 0.0350 m aqueous solution of fluoride, f − , in mass percentage and in parts per million (ppm). assume the density of the solution is 1.00 g/ml. a. number in percentage %
b. number in ppm .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Express the concentration of a 0.0350 m aqueous solution of fluoride, f − , in mass percentage and i...
Questions in other subjects:

Mathematics, 23.10.2020 03:01


History, 23.10.2020 03:01

Mathematics, 23.10.2020 03:01

History, 23.10.2020 03:01


Business, 23.10.2020 03:01

Chemistry, 23.10.2020 03:01


Social Studies, 23.10.2020 03:01

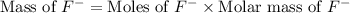
 = 19 g/mole
= 19 g/mole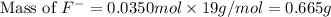
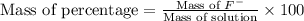
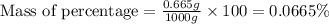
 parts by the mass of the solution.
parts by the mass of the solution.