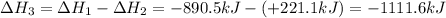Chemistry, 09.12.2019 22:31 santos200154
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 890.5 kj xco 3 ( s ) ⟶ xo ( s ) + co 2 ( g ) δ h = + 221.1 kj what is δ h for this reaction? x ( s ) + 1 2 o 2 ( g ) + co 2 ( g ) ⟶ xco 3 ( s )

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 04:00, Bassoonist
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 890.5 kj xco 3 ( s ) ⟶ xo ( s ) + co...
Questions in other subjects:


Geography, 27.10.2019 23:43




Biology, 27.10.2019 23:43

Mathematics, 27.10.2019 23:43

Computers and Technology, 27.10.2019 23:43


Mathematics, 27.10.2019 23:43

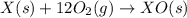
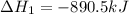 (1)
(1)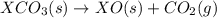
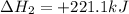 (2)
(2)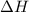 for the following reaction i.e,
for the following reaction i.e,
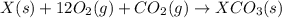
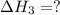 (3)
(3)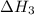 for the reaction will be:
for the reaction will be: