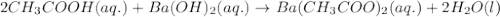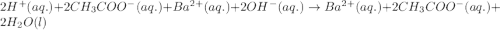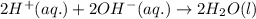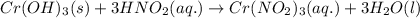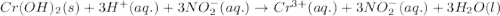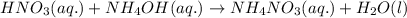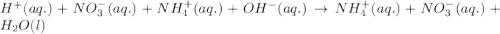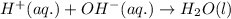Chemistry, 23.11.2019 09:31 sepdentalcare8774
Write the balanced molecular and net ionic equations for each of the following neutralization reactions. (a) aqueous acetic acid (hc2h3o2) is neutralized by aqueous barium hydroxide. (b) solid chromium(iii) hydroxide reacts with nitrous acid. (c) aqueous nitric acid and aqueous ammonia react.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Write the balanced molecular and net ionic equations for each of the following neutralization reacti...
Questions in other subjects:

Biology, 26.07.2019 02:00

History, 26.07.2019 02:00

History, 26.07.2019 02:00

Mathematics, 26.07.2019 02:00


Health, 26.07.2019 02:00




Mathematics, 26.07.2019 02:00

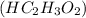 is neutralized by aqueous barium hydroxide.
is neutralized by aqueous barium hydroxide.