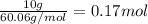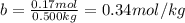Chemistry, 09.12.2019 20:31 alex12everett
The molal freezing point depression constant =kf6.19·°c·kgmol−1 for a certain substance x . when 10.g of urea nh22co are dissolved in 500.g of x , the solution freezes at −6.8°c . calculate the freezing point of pure x . be sure your answer has the correct number of significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


Chemistry, 23.06.2019 07:00, phancharamachasm
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
You know the right answer?
The molal freezing point depression constant =kf6.19·°c·kgmol−1 for a certain substance x . when 10....
Questions in other subjects:



Spanish, 02.10.2019 04:00

Mathematics, 02.10.2019 04:00





English, 02.10.2019 04:00

Mathematics, 02.10.2019 04:00