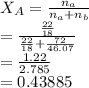Chemistry, 09.12.2019 19:31 historyfanboy101
The vapor pressure of pure water at 15 °c is 12.8 mm hg. what is the equilibrium vapor pressure of water above a mixture of 72.0 g ethanol (ch3ch2oh, molar mass = 46.07 g/mol) and 22.0 g water?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


You know the right answer?
The vapor pressure of pure water at 15 °c is 12.8 mm hg. what is the equilibrium vapor pressure of w...
Questions in other subjects:

History, 21.08.2019 23:50

Physics, 21.08.2019 23:50

Health, 21.08.2019 23:50


Social Studies, 22.08.2019 00:00


Mathematics, 22.08.2019 00:00

Mathematics, 22.08.2019 00:00

Chemistry, 22.08.2019 00:00

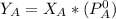
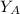 is the partial vapour pressure ( mm Hg)
is the partial vapour pressure ( mm Hg)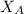 is the mole fraction
is the mole fraction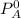 is the pure vapour pressure = 12.8 mm Hg
is the pure vapour pressure = 12.8 mm Hg