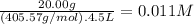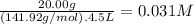Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
What is the molarity of each ion present in aqueous solutions prepared by dissolving 20.00 g of the...
Questions in other subjects:

Mathematics, 03.03.2021 06:40



English, 03.03.2021 06:40


Mathematics, 03.03.2021 06:40

Mathematics, 03.03.2021 06:40

Mathematics, 03.03.2021 06:40