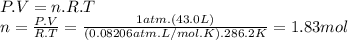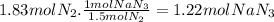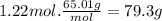The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large volumes of nitrogen gas. 1. write a balanced chemical equation, including physical state symbols, for the decomposition of solid sodium azide ( nan3 ) into solid sodium and gaseous dinitrogen. 2. suppose 43.0l of dinitrogen gas are produced by this reaction, at a temperature of 13.0°c and pressure of exactly 1atm . calculate the mass of sodium azide that must have reacted. round your answer to 3 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

You know the right answer?
The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of...
Questions in other subjects:



History, 16.12.2020 15:30

Mathematics, 16.12.2020 15:30

Arts, 16.12.2020 15:30



Mathematics, 16.12.2020 15:40

Mathematics, 16.12.2020 15:40