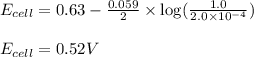Chemistry, 09.12.2019 19:31 LikeIke9418
The standard cell potential (e°cell) for the reaction below is +0.63 v. the cell potential for this reaction is when the concentration of zn2+ = 1.0 m and theconcentration of pb2+ = 2.0 x 10-4 m. pb2+(aq) + zn(s) > zn2+(aq) + pb(s) include details of how to calculate the need the steps. in advance.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1


Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +0.63 v. the cell potential for this...
Questions in other subjects:

Mathematics, 22.02.2021 08:10


Mathematics, 22.02.2021 08:10

English, 22.02.2021 08:10


Mathematics, 22.02.2021 08:10


Biology, 22.02.2021 08:10


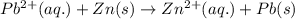
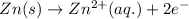
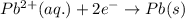
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Zn^{2+}]}{[Pb^{2+}]}](/tpl/images/0410/3244/a3f7c.png)
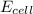 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V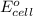 = standard electrode potential of the cell = 0.63 V
= standard electrode potential of the cell = 0.63 V![[Zn^{2+}]=1.0M](/tpl/images/0410/3244/26443.png)
![[Pb^{2+}]=2.0\times 10^{-4}M](/tpl/images/0410/3244/3bdd9.png)