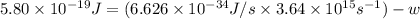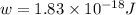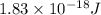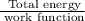Chemistry, 09.12.2019 18:31 gajdmaciej9502
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a kinetic energy of 5.80× 10–19 j. what is the maximum number of electrons that could be ejected from this metal by a burst of light (at some other frequency) with a total energy of 8.66× 10–7 j?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1


Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a ki...
Questions in other subjects:


English, 07.05.2021 03:50




Chemistry, 07.05.2021 03:50

Mathematics, 07.05.2021 03:50

Mathematics, 07.05.2021 03:50

Mathematics, 07.05.2021 03:50

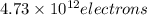
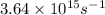
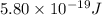
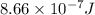
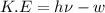
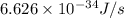
 = frequency
= frequency